- Research
- Open access
- Published:
Acquisition of neurodegenerative features in isogenic OPTN(E50K) human stem cell-derived retinal ganglion cells associated with autophagy disruption and mTORC1 signaling reduction
Acta Neuropathologica Communications volume 12, Article number: 164 (2024)
Abstract
The ability to derive retinal ganglion cells (RGCs) from human pluripotent stem cells (hPSCs) has led to numerous advances in the field of retinal research, with great potential for the use of hPSC-derived RGCs for studies of human retinal development, in vitro disease modeling, drug discovery, as well as their potential use for cell replacement therapeutics. Of all these possibilities, the use of hPSC-derived RGCs as a human-relevant platform for in vitro disease modeling has received the greatest attention, due to the translational relevance as well as the immediacy with which results may be obtained compared to more complex applications like cell replacement. While several studies to date have focused upon the use of hPSC-derived RGCs with genetic variants associated with glaucoma or other optic neuropathies, many of these have largely described cellular phenotypes with only limited advancement into exploring dysfunctional cellular pathways as a consequence of the disease-associated gene variants. Thus, to further advance this field of research, in the current study we leveraged an isogenic hPSC model with a glaucoma-associated mutation in the Optineurin (OPTN) protein, which plays a prominent role in autophagy. We identified an impairment of autophagic-lysosomal degradation and decreased mTORC1 signaling via activation of the stress sensor AMPK, along with subsequent neurodegeneration in OPTN(E50K) RGCs differentiated from hPSCs, and have further validated some of these findings in a mouse model of ocular hypertension. Pharmacological inhibition of mTORC1 in hPSC-derived RGCs recapitulated disease-related neurodegenerative phenotypes in otherwise healthy RGCs, while the mTOR-independent induction of autophagy reduced protein accumulation and restored neurite outgrowth in diseased OPTN(E50K) RGCs. Taken together, these results highlighted that autophagy disruption resulted in increased autophagic demand which was associated with downregulated signaling through mTORC1, contributing to the degeneration of RGCs.
Introduction
Retinal ganglion cells (RGCs) are the sole projection neurons that connect the eye with the brain, and their degeneration in diseases such as glaucoma results in vision loss or blindness [1, 2]. Much of what we currently know about the mechanisms of diseases in the retina has been derived primarily from rodent models [3,4,5]. However, important differences exist between rodent and human RGCs, including a low degree of conservation of RGC subtypes [6,7,8]. As such, human pluripotent stem cell (hPSC) models provide a robust platform to study cellular mechanisms underlying disease states [9]. To model glaucoma neurodegeneration in a human context, we and others have developed human RGCs derived from hPSCs as a translationally relevant resource [10,11,12,13,14]. While hPSC-derived RGCs are considered to represent a somewhat immature, developmental stage, increasing evidence from multiple neurodegenerative diseases suggests that early alterations during development contribute to the later manifestation of neurodegenerative pathogenesis [15, 16], supporting the use of hPSC-based RGC systems to study at least some mechanistic aspects of glaucomatous neurodegeneration.
Neurodegeneration shares many common mechanisms throughout the central nervous system, including glaucoma in the retina [17]. While neurons such as RGCs depend highly on autophagy, deficits in autophagy have been implicated in multiple neurodegenerative diseases [18,19,20,21]. The attenuation of autophagy has also been described in glaucoma in both the trabecular meshwork and RGCs [22,23,24]. Furthermore, a subpopulation of glaucoma patients possess mutations in the autophagy receptor Optineurin (OPTN) or the autophagy-associated protein TANK-binding kinase 1 (TBK1), resulting in glaucoma within a normal range of intraocular pressure [25,26,27]. Despite this, our knowledge of how autophagy impairment promotes neurodegeneration within RGCs, particularly in the human context, remains limited.
We have previously demonstrated that hPSC-derived RGCs can exhibit neurodegenerative phenotypes with a glaucoma-associated OPTN(E50K) mutation [11, 12], yet there have been no prior reports exploring detailed cellular mechanisms explaining how this glaucoma-associated mutation results in neurodegeneration. Importantly, since the OPTN protein serves a primary role as an autophagy receptor [28,29,30], this hPSC-derived cellular model can serve as a robust tool to examine how autophagy dysfunction results in neurodegenerative phenotypes. To address this gap and further explore how autophagy dysfunction adversely affects RGCs, we have advanced our hPSC-RGC neurodegeneration model with an underlying OPTN(E50K) mutation and identified a disruption in OPTN protein processing along with a decrease in autophagic flux in hPSC-RGCs. Interestingly, downregulation of mTORC1 signaling was observed in hPSC-RGCs with the OPTN(E50K) mutation, suggesting that RGC neurodegeneration may partly result from the imbalanced activity of mTOR-dependent autophagy. To test this idea, we found that inducing autophagy in an mTOR-independent manner rescued autophagy deficits and neurodegenerative phenotypes in hPSC-RGCs, suggesting that autophagy dysfunction promotes RGC neurodegeneration at least partially through inhibition of mTORC1 signaling and that modulating these pathways can rescue some neurogenerative phenotypes.
Materials and methods
Human pluripotent stem cell culture and CRISPR/cas9 gene editing
For all studies, we used both the H7 human embryonic stem cell line as well as a patient-derived induced-pluripotent stem cell (iPS) cell line with the OPTN(E50K) mutation. Both of these were previously subjected to gene editing using CRISPR/Cas9 techniques to either introduce the OPTN(E50K) mutation or correct the mutation, respectively, as previously described in Vanderwall and Huang et al. [12]. Additionally, for some experiments, hPSC lines used were previously edited to express a BRN3B-tdTomato-Thy1.2 reporter [12], based upon strategies developed by Sluch et al. [14]. Conversely, for experiments related to the use of the LC3-RFP-GFP sensor in which the tdTomato reporter would interfere with analyses, isogenic pairs of cells were edited that lacked the BRN3b-tdTomato-Thy1.2 reporter following methods previously described [12], and these cell lines were further verified by Sanger sequencing. To maintain all lines of hPSCs, cells were grown on a Matrigel (Corning, cat. no. 354277) coated plate with mTeSR1 medium (StemCell Technologies, cat. no. 85850). hPSCs were passaged with dispase (2 mg/mL, Life Technologies, cat. no. 17105041) when they reached approximately 70%-80% confluency, every 5–7 days.
Differentiation of human RGCs
Human retinal organoids were differentiated following previously published methods [31,32,33,34,35], and RGCs were subsequently purified and grown in culture to induce further maturation as described previously [10, 12, 36]. Briefly, colonies of undifferentiated hPSCs at 80% confluency were enzymatically detached from the plate with dispase and cultured in suspension to induce the formation of embryoid bodies (EBs). EBs were gradually transferred from mTeSR1 to neural induction medium (NIM), which consisted of DMEM/F12(1:1) with N2 supplement, MEM non-essential amino acids and heparin (2 µg/ml). On day 6, BMP4 (50 ng/mL) was added to differentiating cultures, and EBs were subsequently plated on day 8 by supplementation with 10% FBS at a density of approximately 100–200 EBs per well of a 6-well plate to induce a primitive retinal fate. Full medium was changed with NIM on days 9, 12 and 15 and then on day 16, optic vesicle-like structures were lifted and cultured in suspension to induce retinal organoid formation in retinal differentiation medium (RDM), which consisted of DMEM/F12(3:1) supplemented with 2% B27, MEM non-essential amino acids, and penicillin/streptomycin. From day 20–35, half media changes were performed every 2–3 days, and cultures were gradually supplemented with FBS transitioning from 1 to 10%. On day 35, retinal organoids were cultured in retinal maturation medium, consisting of DMEM/F12(3:1) supplement with 2% B27 supplement, MEM non-essential amino acids, penicillin/streptomycin, 10% FBS, 1X GlutaMAX, and 100 μM Taurine. Half media changes were then performed every 2–3 days.
To isolate RGCs, retinal organoids between 45 and 55 days of differentiation were enzymatically dissociated with AccuMax and purified by Magnetic Activated Cell Sorting (MACS) with CD90.2 (Thy1.2) MicroBeads (Miltenyi Biotec, cat. no. 130–121–278) as previously described [14]. Purified RGCs were plated on either poly-D-ornithine and laminin-coated glass coverslips or laminin-coated culture plates at a density of 450 cells/mm2 and maintained in Brainphys medium supplemented with 2% B27 (either with or without insulin, depending on experiment, Life Technologies catalog 17504044 or A1895601, respectively), 20 ng/mL BDNF, 20 ng/mL GDNF, 1 mM dibutyryl cAMP, 200 nM ascorbic acid and penicillin/streptomycin. Half media changes were performed every 3–4 days and maintained up to an additional 4 weeks. For chloroquine treatment, 30 nM chloroquine (Life Technologies, cat. no. P36239B) was added to hPSC-RGCs after 4 weeks of maturation for a duration of 16 h prior to fixation and immunocytochemistry. For mTOR inhibition, the dual mTOR inhibitor KU-0063794 (Tocris, cat. no. 3725) was added at indicated concentrations at 3 days following the isolation of RGCs for an additional week. For RGC neuroprotection, 25 mM trehalose (MP Biomedicals, cat. no. 103097) was dissolved in the medium, filtered, and added to RGCs from week 2 to week 4 following purification.
Differentiation of human cortical neurons
To derive cortical neurons from hPSCs, maintenance, passaging, as well as EB formation were performed as described above for retinal organoids with minor modifications [31]. Primarily, on day 6 of differentiation, 200 nM LDN-193189 (Reprocell, cat. no. 04–0074-02) was added to EBs instead of BMP4. At day 16, the loosely attached rosettes were mechanically lifted and cultured in suspension in RDM as cortical organoids. By day 45, cortical organoids were then enzymatically dissociated with AccuMax and purified by MACS using anti-PSA-NCAM MicroBeads (Miltenyi Biotec, cat. no. 130–097–859) and plated onto poly-D-ornithine and laminin-coated coverslips or laminin-coated culture plates and further maintained in Brainphys medium, as described above.
Magnetic microbead occlusion mouse glaucoma model
All animal procedures were approved by the University of Montreal Hospital Research Centre and followed the Canadian Council on Animal Care guidelines. Experiments were performed in adult female C57B6L/6 mice (2 to 3 months of age, 20.3 to 25.1 g) (Charles River, Strain code:027). All procedures were performed under general anesthesia (20 mg/kg ketamine, 2 mg/kg xylazine, 0.4 mg/kg acepromazine). Unilateral elevation of intraocular pressure was performed by a single injection of magnetic microbeads into the mouse anterior chamber [37]. Animals were anesthetized and a drop of tropicamide was applied on the cornea to induce pupil dilation (Mydriacyl, Alcon, Mississauga, ON, Canada). A custom-made sharpened microneedle attached to a microsyringe pump (World Precision Instruments, Sarasota, FL) was loaded with a magnetic microbead solution (1.5 µl, diameter: 4.5 µm, 2.4 × 106 beads, Dynabeads M-450 Epoxy, ThermoFisher Scientific, Waltham, MA). Using a micromanipulator, the tip of the microneedle was gently pushed through the cornea to inject the microbeads into the anterior chamber. The microbeads were immediately attracted to the iridocorneal angle using a hand-held magnet. This procedure avoided injury to ocular structures including the lens and iris. Sham-operated controls received an injection of phosphate buffered saline (PBS). Only one eye was operated on and an antibiotic drop (Tobrex, tobramycin 0.3%, Alcon) was applied immediately after the surgery. The animal was allowed to recover on a heating pad. Intraocular pressure was measured in awake animals before and after the procedure, and bi-weekly thereafter always at the same time using a calibrated TonoLab rebound tonometer (Icare, Vantaa, Finland). For this purpose, a drop of proparacaine hydrochloride (0.5%, Alcon) was applied to the cornea and a minimum of 10 consecutive readings were taken per eye and averaged.
RNA extraction and quantitative real-time PCR (qPCR)
Total RNA was collected from RGCs following purification and subsequent maturation for 4 weeks using the PicoPure RNA isolation kit (Life Technologies, cat. no. KIT0204). 1 μg of RNA was used for reverse transcription using the High Capacity RNA-to-cDNA kit (Life Technologies, cat. no. 4387406), and cDNA samples were diluted with nuclease-free water at a 1:2 ratio. Quantitative PCR was performed using the Applied Biosystems StepOnePlus Real-Time PCR System with SYBR Green PCR master mix (Life Technologies, cat. no. 4364344). Primer sets used included: OPTN-forward: GACACGTTACAGATTCACGTGA; OPTN-reverse: ACTGTGCCCGGCCTGTTTTC; β-actin-forward: GCGAGAAGATGACCCAGATC; β-actin-reverse: CCAGTGGTACGGCCAGAGG; GAPDH-forward: CGCTCTCTGCTCCTCCTGTT; GAPDH-reverse: CCATGGTGTCTGAGCGATGT. In addition, a melt-curve analysis was performed immediately after the amplification protocol to verify the specificity of amplification. β-actin and GAPDH were used as endogenous controls to normalize the expression levels. Relative mRNA levels were calculated using the ΔΔCT equation [38]. For some experiments in Fig. 3, qPCR was performed using a custom Taqman Array plate following manufacturer’s instructions.
Immunoblotting
hPSC-RGC samples were collected in M-PER Mammalian Protein Extraction Reagent (Life Technologies, cat. no. 78501) supplemented with a protease and phosphatase inhibitor cocktail (Life Technologies, cat. no. 78440). Alternatively, animals were sacrificed by cervical dislocation and the retinas were immediately dissected out in cold PBS, snap-frozen, and kept at − 80 °C until protein extraction. Proteins were extracted by homogenization of retinas in lysis buffer (Tris 50 mM, EDTA 1 mM, NaCl 150 mM, NP-40 1% v/v, NaVO3 2 mM, NaF 5 mM, Na Deoxycholate 0.25%). The homogenized protein samples were combined with 4 × sample buffer and 25 μM DTT and incubated at 100 °C for 10 min followed by loading into a 4–15% gradient pre-cast gel and transferred onto a nitrocellulose membrane through the Trans-Blot Turbo system (BioRad). The membrane was blocked in 5% milk in tris buffered saline (TBS) supplemented with 0.1% Tween-20 (TBS-T) for 30 min. The membrane was then incubated with diluted primary antibodies in TBS-T with 5% BSA overnight at 4 °C. The membrane was washed 3 times with TBS-T on the following day, and the appropriate secondary antibody in 5% milk in TBS-T was applied for an hour at room temperature, protected from light. The membrane was subsequently washed 3 times with TBS-T and imaged using the Li-COR Odyssey CLx imaging system. Protein intensities were quantified for each band and normalized to an internal control (β-actin) using ImageJ. Detailed information regarding primary antibodies as well as the respective dilutions are provided in the Supplemental Table 1.
Immunostaining
Purified hPSC-RGCs were plated on Poly-D-Ornithine and laminin-coated 12 mm coverslips at a density between 20,000 and 50,000 cells. RGCs were fixed at indicated timepoints with 4% paraformaldehyde for 30 min. Alternatively, animals were anesthetized and transcardially perfused with ice-cold 4% paraformaldehyde (PFA) in PBS. Eyes were immediately collected, post-fixed in PFA, and processed to generate cryosections as previously described [39]. Briefly, the cornea and the lens were removed, and the eye cup was incubated in the same fixative for 2 h at 4 °C. Tissue was further incubated in 30% sucrose overnight, and then frozen in optimal cutting temperature (O.C.T.) compound (Tissue-Tek, Miles Laboratories, Elkhart, IN, USA). Retinal cryosections (16 μm) were collected onto gelatin-coated slides for immunostaining. hPSC-RGCs and retinal cryosections were permeabilized with 0.1% Triton-X for 10 min at room temperature. Following three washes in PBS, RGCs were then blocked in 10% donkey serum and 1% BSA for 1 h at room temperature. Primary antibodies (Supplemental Table 1) were diluted in PBS supplemented with 10% donkey serum and 1% BSA and applied overnight at 4 °C. On the following day, RGCs were washed three times with PBS and incubated with secondary antibodies diluted in 10% donkey serum and 1% BSA for 1 h at room temperature. RGCs were then washed three times with PBS and the coverslips mounted onto slides for imaging. Imaging was performed using either a Nikon A1R Confocal Microscope or a Leica DM5500 fluorescence microscope.
Quantification of OPTN puncta was performed in Fiji where particles were analyzed with proper threshold. For LC3 and OPTN colocalization analysis, the LC3B-GFP Sensor (Life Technologies, cat. no. P36235) was added to hPSC-RGCs for 16 h. RGCs were then stained with OPTN and RFP antibodies, with the latter used to enhance the tdTomato signal. The colocalization between LC3 and OPTN puncta was analyzed within RGC somas by using Fiji with the JACop plugin with threshold.
Autophagosome maturation assay
To analyze autophagic flux, hPSCs with the OPTN(E50K) mutation as well as isogenic controls both lacking the BRN3b-tdTomato-Thy1.2 reporter were used. Differentiated retinal organoids were enzymatically dissociated with AccuMax and plated on 12 mm coverslips in Brainphys medium for 4 weeks, as described above. Dissociated cells were treated with 1 μM of 5-fluoro-2′-deoxyuridine (Sigma, cat. no. F0503) for the first 24 h to remove presumptive progenitor and/or glial cells. The RFP-GFP-LC3B sensor (Life Technologies, cat. no. P36239) was added to hPSC-RGCs after 4 weeks of maturation for a duration of 16 h. Subsequently, immunocytochemistry was performed to stain the cells with a BRN3 primary antibody to definitively identify RGCs, followed by an Alexa Fluor 647 anti-goat secondary antibody. Immunofluorescence images were visualized on a Nikon A1R Confocal Microscope with Z-stack. To ensure the proper selection of RGCs, only cells expressing BRN3 were considered for further analysis of RFP-GFP-LC3B expression. Quantification of autophagosomes (both RFP and GFP puncta) and autolysosomes (RFP positive, GFP negative puncta) was performed on RGC somas through Fiji by using JACop plugin with appropriate threshold. Autophagosomes were calculated as the fraction of RFP puncta overlapping with GFP puncta.
Neurite tracing and analysis
hPSC-RGCs or cortical neurons were maintained on 12 mm coverslips. To identify neurites from individual BRN3-tdTomato (RGCs) or β-III Tubulin (cortical neurons) cells, cultures were transfected either with a GFP plasmid using Lipofectamine Stem Reagent (Life Technologies, cat. no. STEM00003) or BacMam GFP (Life Technologies, cat. no. B10383), which provided a relatively low efficiency of transfection that allowed for facile analysis of individual neurons within otherwise dense neuronal cultures that allowed for greater viability of neurons. Transfection was performed two days before fixation, and immunocytochemistry was then performed on these cultures as indicated. Images were taken using a Leica DM5500 fluorescence microscope. Neurite tracing and Sholl analyses were performed using Fiji and the neuroanatomy plugin.
Proteomic analysis by mass spectrometry
Four biological replicates of wild-type and OPTN(E50K) hPSC-RGCs were lysed with 250 µl of 8 M urea in 100 mM Tris HCl, pH 8.5. Following Bradford assay for protein quantitation (Protein Assay Dye Reagent Concentrate, Bio-Rad, Cat No: 5000006), 40 µg of each protein sample was reduced with 5 mM tris (2-carboxyethyl) phosphine hydrochloride (TCEP, Sigma-Aldrich, Cat No: C4706) and then diluted in 50 mM Tris HCl. Samples were then digested overnight at 35ºC using Trypsin/Lys-C (Trypsin/Lys-C Mix, Mass Spec Grade, Promega, Cat No: V5072); the enzyme–substrate ratio of 1:70). Digestions were quenched with trifluoracetic acid (TFA, 0.5% v/v) and desalted on Waters Sep-Pak cartridges, followed by elution in 50% and 70% acetonitrile containing 0.1% formic acid (FA), and then dried and stored at -20ºC. Dried peptides were later reconstituted in 50 mM triethylammonium bicarbonate pH 8.0 (TEAB). Peptides were labeled for two hours at room temperature with 0.2 mg of Tandem Mass Tag (TMT) reagent (TMT™ Isobaric Label Reagent Set, Thermo Fisher Scientific, Cat No: 90110, Lot No: WG320953). Labeled peptides were then pooled and dried, and then reconstituted in 0.1% TFA and half was fractionated using a Waters Sep-Pak cartridge (Waters™, Cat No: WAT054955) with a wash of 1 mL 0.1% TFA and 0.5% acetonitrile containing 0.1% triethylamine followed by elution in 10%, 12.5%, 15%, 17.5%, 20%, 22.5%, 25% and 70% acetonitrile containing 0.1% triethylamine. A tenth of each fraction was separated on a 25 cm aurora column (IonOpticks, AUR2-25075C18A) at 400 nL/min in the EASY-nLC HPLC system (SCR: 014993, Thermo Fisher Scientific). Nano-LC–MS/MS data were acquired in Orbitrap Eclipse™ Tribrid mass spectrometer (Thermo Fisher Scientific) with a FAIMS pro interface. The data were recorded using Thermo Fisher Scientific Xcalibur (4.3) software (Thermo Fisher Scientific Inc.). Data is available at ProteomeXchange, Accession number PXD033173.
Glucose uptake metabolic assay
Metabolic differences between isogenic control and OPTN(E50K) RGCs were determined through the use of a Glucose Uptake Assay Kit (Abcam 136955), following manufacturer’s instructions. Briefly, RGCs were incubated with Krebs–Ringer-Phosphate-Hepes (KRPH) buffer with 2% BSA for 40 min to induce cell starvation. As some of our experiments examined the role of insulin in RGC homeostasis, we excluded insulin in these assays contrary to manufacturer’s directions. Subsequently, after starvation, RGCs were incubated with 1 mM 2-Deoxyglucose. Cells were then washed three times with PBS and lysed with the extraction buffer. After a cycle of freeze/thaw, cell lysates were incubated at 85 °C for 40 min, neutralized with neutralization buffer and centrifuged at 500 rpm for 2 min. Supernatants were then incubated with reaction mix A (NADPH generation) for 1 h at 37 °C. Next, 90 µl of extraction buffer was added to each sample and incubated for 40 min at 90 °C. Samples were then neutralized with neutralization buffer, incubated with reaction mix B (recycling amplification reaction) and the absorbance was measured at OD 412 nm using the BioTek Synergy H1 microplate reader. Results are represented as the concentration (µM) of 2-Deoxyglucose uptaken by RGCs.
Statistical analysis
Data in all experiments is represented as mean ± SEM and n represents the number of technical replicates across all experiments. Statistical comparisons were conducted by either Student's t-test or ANOVA with Tukey post hoc test (specified in figure legends) using GraphPad Prism 9. Statistically significant differences were defined as p < 0.05 in all experiments including proteomics. For proteomics data analysis, RAW files were analyzed in Proteome Discover™ 2.5 (Thermo Fisher Scientific, RRID: SCR_014477) with a Homo sapiens UniProt reviewed FASTA and common contaminants (20417 total sequences). Normalized abundance values for each sample type, abundance ratio, log2(abundance ratio) values, and respective p-values (t-test) from Proteome Discover™ were exported to Microsoft Excel. All processed and raw data are uploaded to ProteomeXchange Accession PXD033173.
Results
The glaucoma associated OPTN(E50K) mutation alters endogenous protein modification and leads to protein accumulation in hPSC-RGCs
We have previously established a human patient-specific induced pluripotent stem cell (iPSC) line with an OPTN(E50K) mutation, as well as an isogenic control cell line through the CRISPR/Cas9 correction of the mutation. Similarly, we generated a second isogenic pair by introducing the OPTN(E50K) mutation into an unaffected H7 hPSC line carrying an RGC-specific BRN3b-tdTomato-Thy1.2 reporter [12]. Both OPTN(E50K) lines exhibited neurite retraction and hyperexcitable phenotypes [12], although the mechanisms leading to these changes were unclear. In the current study, we approached each set of analyses with these two isogenic pairs of cell lines including OPTN(E50K) and unaffected control (wild-type) RGCs to minimize genetic variability among comparisons, and analyses were typically performed at stages of RGC maturation at which they had been previously demonstrated to exhibit disease-associated phenotypes [12].
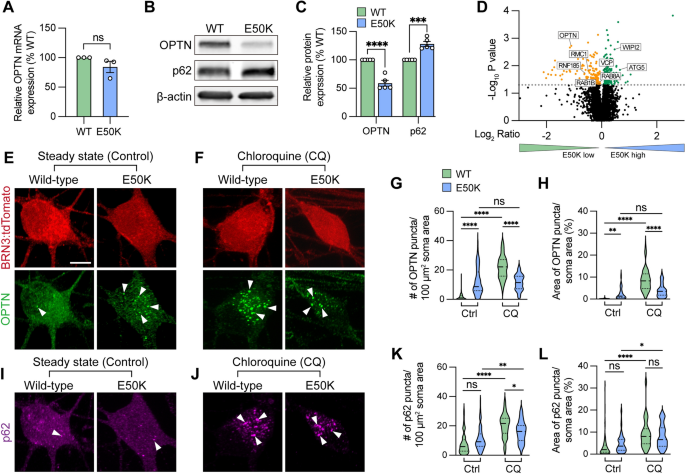
To study how the endogenous OPTN(E50K) mutation attenuates RGC homeostasis, we first characterized the expression of OPTN. While the level of OPTN mRNA was not significantly changed among wild-type and OPTN(E50K) RGCs, there was a 41.1 ± 5.2% (mean ± SEM.) reduction in the level of OPTN protein in RGCs with the OPTN(E50K) mutation, along with a 28.1 ± 4.3% increase in the autophagy receptor p62 (Fig. 1A–C), consistent with previous findings that the E50K mutation decreased the overall abundance of OPTN protein in the mouse eye [40]. To verify these results, we also referenced our previously obtained RNA-seq data [12], which confirmed the lack of changes in expression at the transcriptional level observed in our qRT-PCR results. These results indicated that the E50K mutation likely altered OPTN protein expression through post-translational modifications, and the increased level of p62 may be associated with autophagic accumulation. To ensure that this difference in OPTN protein was not due to changes in solubility, we performed Western blots of both the soluble and insoluble fractions, which both demonstrated significant decreases in OPTN protein (Figure S1). To rule out the possibility that the reduction of OPTN protein was due to a decrease in the specificity of the antibody due to the E50K mutation, we performed an unbiased proteomics analysis of isogenic control and OPTN(E50K) RGCs two weeks after purification and identified 154 downregulated proteins as well as 178 upregulated proteins (Fig. 1D). Among the downregulated proteins, OPTN was identified with 4 peptides and 5 peptide-spectrum matches (PSM), and levels were significantly decreased in OPTN(E50K) RGCs, corroborating our Western blot results. Of interest, we identified an additional 7 autophagy-associated proteins whose expression was also altered, suggesting further disruption of the autophagy pathway due to the OPTN(E50K) mutation (Fig. 1D). Interestingly, proteomics data also indicated misregulation of several ubiquitination-associated proteins, including USP14, UBE2D1, ARIH1, HUWE1, UBA52, and BRCC3, while analyses of Biological Processes demonstrated a misregulation of proteins involved in processes such as protein transport, mRNA processing, and autophagy, while Pathway Analyses suggested changes associated with numerous neurodegenerative diseases (e.g. ALS, Parkinson’s, Huntington, etc.) as well as changes in pathways associated with the proteosome and spliceosome (Figure S2). These analyses of proteomics results also demonstrated the misregulation of numerous proteins associated with mitochondrial or endoplasmic reticulum function, providing the possibility that some differences in OPTN protein expression may be associated with changes in cellular processes such as ubiquitination and subsequent processing, as well as autophagy and mitophagy.
The glaucoma associated OPTN(E50K) mutation alters endogenous protein modification and aggregates in hPSC-RGCs. A Real-time RT-PCR quantification of OPTN mRNA levels (n = 3 for each WT and E50K; t-test, p = 0.181). B–C Western blot and the relative protein expression of OPTN and p62 to β-actin in hPSC-RGCs (n = 5 for each WT and E50K; t-test, OPTN ****p < 0.0001, p62 ***p = 0.0002). D Proteomics analysis demonstrated changes in the expression of autophagy-associated proteins in OPTN(E50K) hPSC-RGCs. E–F Immunostaining displayed the expression of OPTN puncta in BRN3:tdTomato hPSC-RGCs from WT and E50K under steady state (control) and chloroquine (CQ) treatment. Arrows indicate the OPTN puncta. Scale bar: 10 μm. G–H Quantification of OPTN puncta in hPSC-RGCs. I–J Immunostaining displayed the expression of p62 puncta in BRN3:tdTomato hPSC-RGCs from WT and E50K under control and CQ treatment. K–L Quantification of p62 puncta in hPSC-RGCs. G–H and K–L: n = 3 biological replicates using Ctrl-WT n = 60, Ctrl-E50K n = 60, CQ-WT n = 57 and CQ-E50K n = 48 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns = not significant, p > 0.05). Data are represented as mean values ± SEM
Under homeostatic conditions, the number of autophagosomes is balanced between autophagic biogenesis and degradation by the lysosome. Wild-type RGCs showed a predominantly cytosolic OPTN pattern with few puncta observed in RGCs (Fig. 1E), similar to patterns observed previously [41]. However, we observed that OPTN(E50K) RGCs displayed a significant increase in OPTN puncta within RGC somas, indicating an abnormal phenotype (Fig. 1E). To measure any preliminary changes in autophagic biogenesis without the loss of any autophagosomes due to lysosome-mediated degradation, we inhibited autophagosome-lysosome fusion by treatment with chloroquine for 16 h prior to fixation, as previously described [42], where we found that the number of OPTN puncta were significantly decreased in OPTN-E50K RGCs compared to control RGCs following chloroquine treatment (Fig. 1F). More intriguing, however, were the comparisons made not only between control and OPTN-E50K RGCs within each treatment group, but those comparisons across treatment groups (Fig. 1G–H). Indeed, the treatment of control RGCs significantly increased the number of OPTN puncta, supporting the idea of chloroquine inhibiting autophagic flux. However, when OPTN-E50K RGCs were treated with chloroquine, there was no significant change in the number of OPTN puncta observed compared to untreated OPTN-E50K RGCs, supporting the idea that the OPTN-E50K mutation itself either already inhibited autophagic flux or altered OPTN protein structure that accumulated under homeostasis condition (Fig. 1E–H). Additionally, no difference was observed in another autophagy receptor p62 puncta abundance between wild-type and OPTN(E50K), as the puncta were significantly increased after chloroquine treatment both wild-type and OPTN(E50K) RGCs, indicating that the E50K mutation did not affect p62 protein processing (Fig. 1I–L). To rule out the possibility that the genomic background of the cell line caused this phenotype, including silent mutations introduced during CRISPR/Cas9 genome editing [12], we performed the same experiments and observed the same trends in RGCs derived from another OPTN(E50K) iPSC line in comparison with its genome-corrected isogenic control (Figure S3). Collectively, these findings suggest that the E50K mutation adversely affected RGCs through the accumulation of OPTN protein, potentially contributing to RGC neurodegeneration.
OPTN(E50K) hPSC-RGCs display autophagosome accumulation and impaired autophagic-lysosomal degradation
During the process of autophagy, OPTN is necessary to recruit the microtubule-associated protein light chain 3 (LC3) for the engulfment of protein aggregates and/or damaged organelles by formation of the autophagosome [29, 43]. To examine whether this OPTN functional recruitment changes due to the E50K mutation in RGCs, we first used a GFP-fused LC3 reporter to visualize the co-localization of LC3 and OPTN. We found that 10.7 ± 1.2% of LC3 puncta co-localized with OPTN in wild-type RGCs, while only 5.1 ± 0.6% co-localized in RGCs with the OPTN(E50K) mutation (Fig. 2A–I), suggesting that the E50K mutation attenuated OPTN recruitment of LC3. Inhibition of lysosome-mediated degradation by chloroquine demonstrated a similar outcome (Figure S4). We observed no difference in the cytosolic LC3-I level in OPTN(E50K) RGCs (Fig. 2J, K), but a significant increase in the lipidated form of LC3-II, an indicator of autophagosome abundance [44], as well as autophagic flux as determined by the ratio of LC3-II/LC3-I (Fig. 2J–L). Importantly, lysosomal protein LAMP1 expression was also upregulated in OPTN(E50K) RGCs (Fig. 2J, K), suggesting that the OPTN(E50K) mutation not only induced autophagosome accumulation but also changed lysosomal degradation.
OPTN(E50K) hPSC-RGCs displays autophagosome accumulation and impairs autophagic-lysosomal degradation. A–I Analysis of protein colocalization between OPTN and LC3 in hPSC-RGCs (n = 3 biological replicates using WT (n = 36) and E50K (n = 40); t-test, ****p < 0.0001). Yellow arrows label the colocalization between OPTN and LC3 puncta, and white arrows represented that the LC3 puncta did not colocalize with OPTN. Scale bar: 10 μm. J–L Western blot and subsequent analysis of relative protein expression demonstrated increased LC3-II, LAMP1 and autophagic flux (LC3-II to LC3-I ratio) in OPTN(E50K) hPSC-RGCs (n = 6 for each WT and E50K; t-test, LC3-I p = 0.41, LC3-II *p = 0.016, LAMP1 **p = 0.0018, LC3-II/I *p = 0.039). M Schematic of LC3-RFP-GFP probe paradigm. N–V Using LC3-RFP-GFP probe showed the accumulation of autophagosome (RFP + GFP +) in OPTN(E50K) hPSC-RGCs (n = 3 biological replicates using WT n = 44 and E50K n = 45 technical replicates; t-test, **p = 0.0075). Scale bar: 10 μm in A, also refers to E; 5 μm in B, also refers to C, D, F–H. Data are all represented as mean values ± SEM
Disruption of the autophagic-lysosomal pathway has been implicated in multiple neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis, leading to a deficit in protein degradation [45,46,47]. Since we observed the upregulation of autophagosome and lysosome proteins in OPTN(E50K) RGCs, we investigated whether these changes occurred in response to perturbations in the autophagic-lysosomal pathway by expressing RFP and GFP fused to LC3 in RGCs (Fig. 2M) [48]. This probe discriminated between the autophagosome and the acidic autolysosome due to differences in pH sensitivity. Both RFP and GFP signals were expressed in the autophagosome, while under acidic conditions within the autolysosome, the RFP signal persisted as the GFP signal was extinguished. As our established hPSC-RGC system previously included a BRN3-tdTomato reporter, whose red fluorescence could interfere the results using this probe, we used CRISPR/Cas9 genome editing to insert the OPTN(E50K) mutation in the H7 hPSC line without the BRN3-tdTomato reporter, and subsequently identified RGCs by staining with an antibody against BRN3 when imaging. In wild-type RGCs, 25.5 ± 2.8% of RFP puncta co-expressed GFP, indicating the remaining 74.5 ± 2.8% of autophagosomes had fused with the lysosome (Fig. 2N–Q, Supplemental Movie S1). However, OPTN(E50K) RGCs exhibited significantly more overlap between RFP and GFP at 35.8 ± 2.5% (Fig. 2R–V, Supplemental Movie S2) and increased the number of GFP puncta but not RFP (Fig. 2V), highlighting that the OPTN(E50K) mutation resulted in an impaired ability of the autophagosome to properly fuse with the lysosome for subsequent degradation.
OPTN(E50K) hPSC-RGCs downregulate mTORC1 signaling
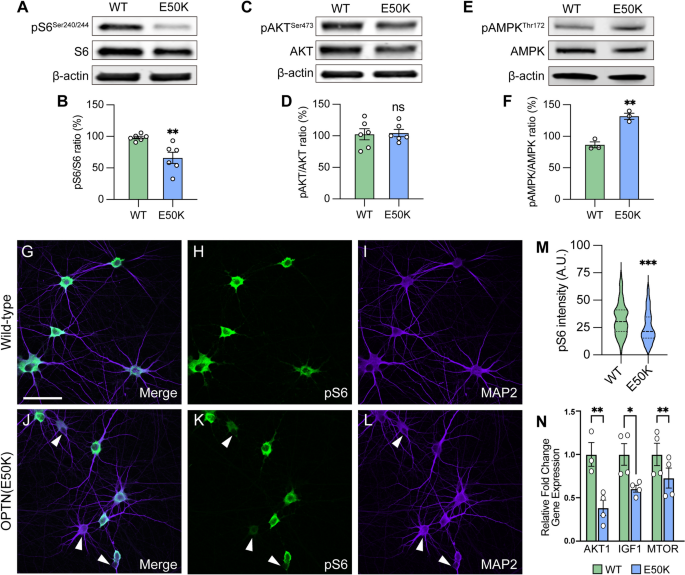
The mammalian target of rapamycin (mTOR) signaling pathway is a key metabolic regulator and sensor of stress. Activation of mTOR is known to promote dendritic morphological complexity and axonal regeneration in RGCs, while also functioning as a negative regulator of autophagy through the mTORC1 complex [49,50,51,52,53]. Under cellular stress in neurons, autophagy disruption can activate adenosine monophosphate-activated protein kinase (AMPK) to induce the repression of mTORC1, resulting in neurodegeneration [54, 55]. To determine whether autophagy disruption due to the OPTN(E50K) mutation induced RGC degeneration associated with mTORC1 signaling, we used pS6Ser240/244 as a readout of mTORC1 activity [56]. We first characterized mTORC1 activity in hPSC-derived retinal cells by isolating cells from retinal organoids after 50 and 80 days of differentiation, respectively, which allowed for the analysis of the majority of neuroretinal cell types including RGCs (BRN3B-tdTomato), retinal progenitors (CHX10), and photoreceptors (OTX2) (Figure S5A–C). Immunocytochemical detection of pS6Ser240/244 revealed a robust expression within BRN3B:tdTomato RGCs, but not in retinal progenitor cells nor photoreceptors, indicating a strong role for mTOR signaling specifically within hPSC-RGCs (Figure S5D, E). Subsequently, we analyzed RGCs that were isolated from retinal organoids and allowed to mature for an additional 4 weeks, a timepoint at which we have previously demonstrated to result in neurodegenerative phenotypes in OPTN(E50K) RGCs [12]. OPTN(E50K) RGCs exhibited a decreased expression of the mTORC1 effector pS6Ser240/244, while no difference was observed in the expression of the mTORC2 effector pAKTSer473 (Fig. 3A–D). More so, OPTN(E50K) RGCs also increased the level of the phosphorylated form of AMPK (pAMPKThr172), which is a stress activator and mTORC1 inhibitor (Fig. 3E, F). Immunocytochemistry also allowed for the more detailed investigation of pS6Ser240/244 within individual RGCs, which revealed a profound decrease in the expression of pS6Ser240/244 intensity in the somatic area of a subset of OPTN(E50K) RGCs (Fig. 3G–M), while others appeared to maintain normal expression levels. To further explore changes in mTOR signaling as a result of the OPTN(E50K) mutation, we then explored prior RNA-seq data comparing isogenic control and OPTN(E50K) RGCs taken after just 10 days of maturation [12], and found a significant reduction in the expression of some other mTOR-associated genes (Fig. 3N). Taken together, our results suggest that chronic autophagy deficits in glaucomatous RGCs were also associated with the activation of AMPK signaling to suppress mTORC1.
Attenuation of mTORC1 signaling in OPTN(E50K) hPSC-RGCs. A–F Western blot and the relative protein expression of pS6Ser240/244, pAKTSer473 and pAMPKThr172 to its total protein, respectively, in hPSC-RGCs (n = 6 for each WT and E50K; t-test, pS6Ser240/244 **p = 0.0057; pAKTSer473 ns = not significant, p = 0.854; pAMPKThr172 **p = 0.0027). G–M Immunostaining and quantification of pS6Ser240/244 intensity revealed that a subset of RGCs with the OPTN(E50K) mutation reduced the expression of pS6Ser240/244 (n = 3 biological replicates using WT n = 96 and E50K n = 96 technical replicates; t-test, ***p = 0.0009). Arrows indicate RGCs that have reduced expression of pS6Ser240/244. N RNA-seq analyses demonstrated a significant reduction in some mTOR-associated genes. Scale bar: 25 μm. Data are all represented as mean values ± SEM
Glaucoma RGCs subjected to ocular hypertensive stress demonstrated autophagy deficits similar to OPTN(E50K) RGCs
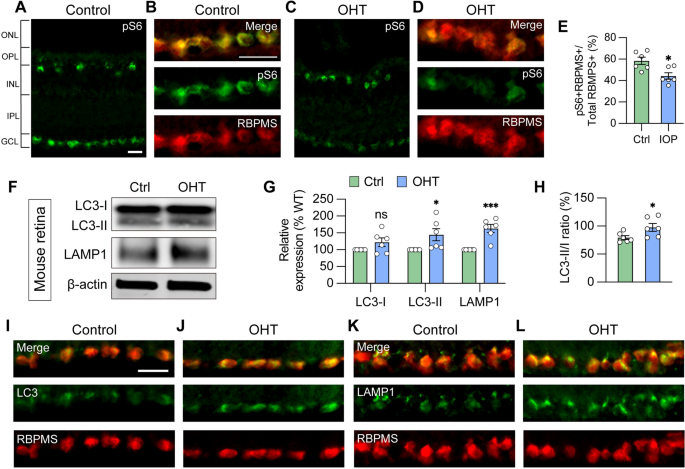
Many underlying causes of glaucoma may exist, including elevated intraocular pressure, environmental factors, and genetic contributions. Despite varying underlying causes, pathological features are often similar across patients, suggesting perhaps that some cellular mechanisms may be consistent. Multiple missense mutations in OPTN or TBK1, known as autophagy regulators, are known to result in normal tension glaucoma [27, 57], suggesting that autophagy homeostasis plays a key role in RGC survival and, conversely, inappropriate autophagy processing may contribute to glaucomatous neurodegeneration. However, it was unclear if the phenotypes observed in OPTN(E50K) RGCs thus far were relevant to other forms of glaucoma. Thus, to determine if the dysfunctional autophagy phenotypes observed in OPTN(E50K) RGCs were similarly identified in other models of glaucoma, thereby suggesting a broader relevance in RGC neurodegeneration, we used a well-established magnetic microbead occlusion glaucoma model to induce ocular hypertension through the injection of magnetic microbeads into the anterior chamber of the mouse eye. This procedure blocks aqueous humor outflow resulting in elevated intraocular pressure (IOP) (Figure S6 A) [37, 58], a major risk factor for the development of glaucoma. Indeed, RGCs from sham-injected control mice exhibited robust mTORC1 activity, as visualized by pS6Ser240/244 co-localization with the RGC marker RBPMS, while decreased mTORC1 activity was observed in RGCs subjected to ocular hypertensive stress (Fig. 4A–E). This observation is in agreement with previous findings that mTORC1 signaling is partially inactivated in glaucomatous RGCs through AMPK phosphorylation resulting in dendritic retraction [58], and also corroborates results obtained with OPTN(E50K) RGCs (Fig. 3G–M).
Glaucoma RGCs subjected to ocular hypertensive stress demonstrate autophagy deficits. A, B Immunostaining labeled the level of the mTORC1 effector pS6Ser240/244 and exhibited robust activity in the ganglion cell layer (GCL), co-labeled with RBPMS in B, and inner nuclear layer (INL) in control mouse retina. Scale bar: 25 μm. C–E Under ocular hypertension, immunostaining and associated quantification demonstrated that the level of pS6Ser240/244 decreased in mouse RGCs (n = 3 mice/group, 2 images/mice; t-test, *p = 0.011). F–H Western blot verified changes in protein expression of LC3-I, LC3-II, LAMP1, and LC3-II/I ratio in control or glaucoma mouse retinas with ocular hypertension (OHT) (n = 6 for each control and OHT; t-test, LC3-I p = 0.122, LC3-II *p = 0.034, LAMP1 ***p = 0.0004, LC3-II/I *p = 0.042). I–L Immunostaining displayed the elevation of LC3 and LAMP1 in RBPMS-expressing RGCs after ocular hypertension. Scale bar: 25 μm. Data are all represented as mean values ± SEM
Subsequently, changes in the expression of autophagy markers were examined 2 weeks after microbead injection, a time that precedes RGC loss and thereby avoids the confounding effect of overt neurodegeneration [58]. A significant increase in LC3-II, the LC3-II/I ratio, and LAMP1 were observed in the retina 2 weeks after ocular hypertension induction compared to sham-injected controls (Fig. 4F–H), in agreement with our results obtained with OPTN(E50K) hPSC-derived RGCs (Fig. 2J–L). Immunostaining of retinal sections further showed the increased expression of LC3 and LAMP1 more specifically in the ganglion cell layer, which co-localized with the RGC marker RBPMS (Fig. 4I–L), similar to results observed by others in mouse models [24]. No changes were observed in the expression of OPTN protein within the OHT and control mouse retinas as determined by Western blot (Figure S7), although this lack of difference could be due to the analysis of total retinal protein rather than purely RGC protein content. Collectively, our findings highlighted that autophagy disruption is associated with RGC neurodegeneration not only in hPSC-RGCs with the OPTN(E50K) mutation, but in an ocular hypertension system, suggesting that autophagy disruption in RGCs may be a common mechanism that contributes to neurodegeneration across multiple glaucoma models.
Selective degeneration of RGCs with the OPTN(E50K) mutation
To determine if the OPTN(E50K) mutation selectively promotes neurodegeneration in RGCs as indicated by its role in glaucoma, or if it can confer effects within other cell types in an in vitro model, we evaluated the effects within two types of neurons– hPSC-RGCs and hPSC-cortical neurons (Figure S6 B), based upon their neurite outgrowth and protein expression profiles, respectively. We confirmed that wild-type and OPTN(E50K) cells were both effectively differentiated from hPSCs in parallel cultures based upon the expression of lineage-associated makers, including of BRN3/MAP2 (RGCs), and CTIP2/bIII-tubulin (cortical neurons) (Fig. 5A, D, respectively). RGCs with the OPTN(E50K) mutation exhibited shorter neurites compared to wild-type, as analyzed by neurite complexity, soma area, number of primary neurites, and total neurite length (Fig. 5 A–C, G–I), concomitant with our previous findings [12]. In contrast, neurites from cortical neurons did not exhibit any significant differences between wild-type and OPTN(E50K) under the same measured parameters (Fig. 5D–I), indicating that neurodegenerative features were selectively identified in RGCs with the OPTN(E50K) mutation.
Selective degeneration of RGCs with the OPTN(E50K) mutation. A Immunostaining to characterize wild-type and OPTN(E50K) RGCs. Scale bar: 25 μm. B Representative neurite tracing of wild-type and OPTN(E50K) RGCs after 4 weeks of purification. Scale bar: 150 μm. C Sholl analysis revealed the neurite complexity in wild-type and OPTN(E50K) RGCs (n = 3 biological replicates using WT n = 15 and E50K n = 17 technical replicates; t-test, ****p < 0.0001). D Immunostaining to characterize wild-type and OPTN(E50K) cortical neurons. E Representative neurite tracing of wild-type and OPTN(E50K) cortical neurons 4 weeks after purification. Scale bar: 150 μm. F Sholl analysis revealed similar neurite complexity in wild-type and OPTN(E50K) cortical neurons (n = 3 biological replicates using WT n = 16 and E50K n = 17 technical replicates; t-test, ns = not significant, p = 0.37). G–I Quantitative analysis of neurite parameters displayed neurite deficits in OPTN(E50K) RGCs based upon measurements of soma area (n = 3 biological replicates using RGC-WT n = 101, RGC-E50K n = 112, cortical-WT n = 68, and cortical-E50K n = 66 technical replicates; t-test, RGC: *** p = 0.0003; cortical: ns = not significant, p = 0.811) G, number of primary neurites (n = 3 biological replicates using RGC-WT n = 15, RGC-E50K n = 17, cortical-WT n = 16, and cortical-E50K n = 17 technical replicates; t-test, RGC: ** p = 0.004; cortical: ns = not significant, p = 0.626) H and total neurite length (n = 3 biological replicates using RGC-WT n = 15, RGC-E50K n = 17, cortical-WT n = 16, and cortical-E50K n = 17 technical replicates; t-test, RGC: **** p < 0.0001; cortical: ns = not significant, p = 0.575) I, but not in OPTN(E50K) cortical neurons when compared with wild-type. J–M Western blot and quantified relative protein expression demonstrated that the OPTN(E50K) mutation altered LC3-II only in RGCs (n = 3 for each WT and E50K from RGC or cortical neurons; One-way ANOVA, Tukey post hoc test. ***p < 0.001, **p < 0.01, *p < 0.05, ns = not significant, p > 0.05). N–U Immunostaining displayed the expression of OPTN puncta in hPSC-derived cortical neurons from WT and E50K under steady state (control) N–Q and chloroquine (CQ) treatment R–U. Scale bar: 10 μm. V, W Quantification of OPTN puncta in hPSC-derived cortical neurons (n = 3 biological replicates using Ctrl-WT n = 39, Ctrl-E50K n = 34, CQ-WT n = 37 and CQ-E50K n = 37 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001, *p < 0.05, ns = not significant, p > 0.05). Data are all represented as mean values ± SEM
We then sought to determine how the OPTN(E50K) mutation affected the expression of some autophagy-associated proteins. The level of OPTN protein decreased with the E50K mutation in both neuronal types (Fig. 5J, K). Additionally, there was a higher cytosolic LC3-I level in RGCs compared to cortical neurons (Fig. 5J, lane 1 and 3), and only OPTN(E50K) RGCs exhibited a significant increase in the lipidated form of LC3-II (Fig. 5J, L, M). Unlike the observed accumulation of OPTN protein in RGCs (Fig. 1E), OPTN puncta did not deposit in cortical neurons with the E50K mutation (Fig. 5N–Q), while the E50K mutation led to overall protein level reduction after chloroquine treatment (Fig. 5R–W). The abundance of p62 puncta was not affected overall in cortical neurons (Figure S8). Collectively, our findings suggest that the OPTN(E50K) mutation selectively rendered RGCs more vulnerable to neurodegeneration, with data suggesting a greater autophagic demand in RGCs that may be associated with their selective degeneration.
Inhibition of mTOR signaling results in neurodegenerative phenotypes in otherwise healthy hPSC-RGCs
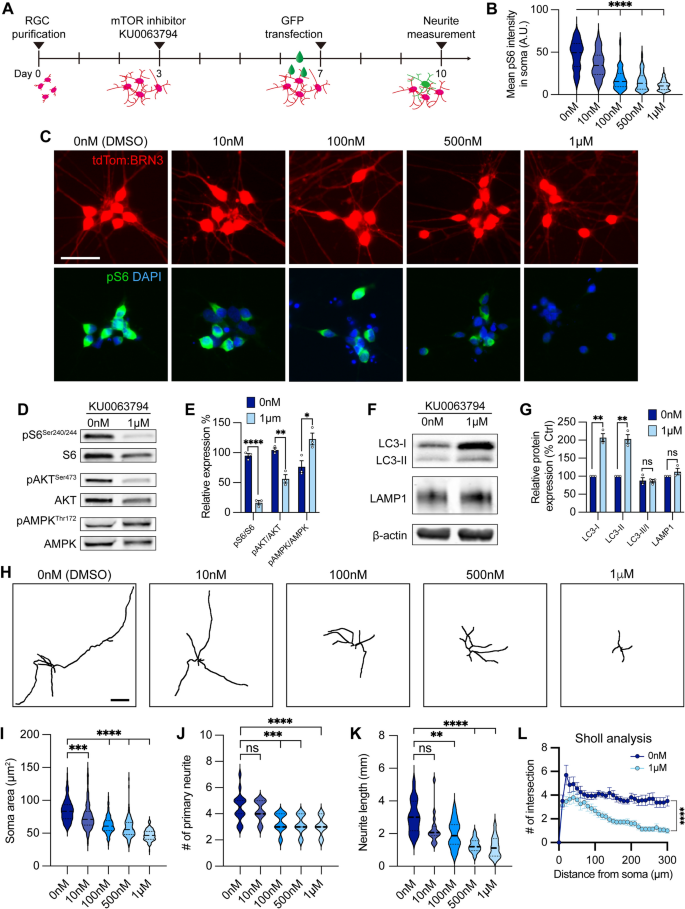
To evaluate whether decreased mTOR activity was associated with impaired RGC neurite outgrowth and autophagy modulation observed in OPTN(E50K) RGCs, we examined how healthy control hPSC-RGCs were affected when treated with the dual mTORC1/2 inhibitor KU0063794 for one week (Fig. 6A). RGCs exhibited a reduction of pS6Ser240/244 intensity following treatment with KU0063794 in a dose-dependent manner (Fig. 6B, C). When treated with KU0063794 (1 µM), RGCs revealed significant decreases in the expression of both mTORC1 and mTORC2 effectors pS6Ser240/244 and pAKTSer473, respectively, while the level of pAMPKThr172 was increased (Fig. 6D, E). Pharmacological inhibition of mTOR also resulted in increased LC3-I and LC3-II, an indication of autophagy activation (Fig. 6F, G). However, the level of autophagic flux (LC3-II/LC3-I) and lysosome protein LAMP1 did not change under mTOR inhibition when compared to vehicle control, suggesting that wild-type RGCs can effectively balance cellular homeostasis between autophagy and acute mTOR inhibition. Analyses of RGC neurites demonstrated a decrease in neurite complexity that correlated with an increase in the concentration of KU0063794 (Fig. 6H–L). As morphological features of RGCs were modulated by KU0063794 in a dose-dependent manner, these results suggest that the pathological hallmarks of neurite retraction observed in OPTN(E50K) RGCs may be the result of progressively decreased mTOR signaling.
mTOR inhibition contributed to neurite shortening in hPSC-derived RGCs. A Schematic timeline of mTOR inhibitor treatment and methods for neurite analyses. B–C Quantification and immunostaining revealed that pS6Ser240/244 intensity was dose-dependent in response to mTOR inhibition in hPSC-RGCs (n = 3 biological replicates using vehicle (DMSO) n = 162, 10 nM n = 143, 100 nM n = 128, 500 nM n = 121, and 1 μM n = 133 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001 for each KU0063794 treatment group compared to vehicle treatment). Scale bar: 25 μm. D–G Western blot and quantification of the relative protein expression displayed a decrease of mTOR signaling and induction of autophagy when mTOR was inhibited by KU0063794 treatment in hPSC-RGCs (n = 3 for each vehicle (DMSO) and 1 μM of KU0063794 treatment, t-test, pS6Ser240/244 ****p < 0.0001, pAKTSer473 **p = 0.0033, pAMPKThr172 **p = 0.0292, LC3-I ***p = 0.0006, LC3-II **p = 0.001, LC3-II/I p = 0.838, LAMP1 p = 0.23). H Representative neurite tracings of vehicle and KU0063794 treatment in hPSC-RGCs. Scale bar: 200 μm. I–L Quantitative analysis of neurite parameters displayed neurite deficits and decreased neurite complexity following mTOR inhibition in hPSC-RGCs as measured by their soma area (n = 3 biological replicates using vehicle n = 107, 10 nM n = 109, 100 nM n = 97, 500 nM n = 107, and 1 μM n = 93 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001) I, number of primary neurites (n = 3 biological replicates using vehicle n = 16, 10 nM n = 16, 100 nM n = 16, 500 nM n = 17, and 1 μM n = 15 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001, ns = not significant, p > 0.05) J, total neurite length (n = 3 biological replicates using vehicle n = 16, 10 nM n = 16, 100 nM n = 16, 500 nM n = 17, and 1 μM n = 15 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, **p < 0.01, ns = not significant, p > 0.05) K, and Sholl analysis (n = 3 biological replicates using vehicle n = 16, and 1 μM n = 15 technical replicates; t-test, ****p < 0.0001) L. Data are all represented as mean values ± SEM
Altered mTOR signaling and autophagy modulated RGC neurodegenerative phenotypes
Insulin is a canonical mTOR activator and previous studies have demonstrated the ability of insulin to rescue neurodegenerative phenotypes in dendritic arbors in rodent RGCs subjected to optic nerve crush [49]. We have demonstrated that OPTN(E50K) RGCs exhibit morphological and functional deficits as soon as 4 weeks after the purification and maturation from retinal organoids (Fig. 5A–C) [12]. However, as insulin is a common component of many cell culture media supplements (such as N2 and B27 supplements), it was present in prior experiments to act upon RGCs and modulate mTOR signaling. Thus, we investigated whether insulin deprivation can lead to a faster disease phenotype in OPTN(E50K) RGCs through a reduced activity of the mTOR signaling pathway. RGCs were grown with medium supplemented with B27 either with or without insulin (normal levels of insulin provided by B27 supplement, or in the complete absence of insulin), and RGC neurites were measured from 1 to 4 weeks of maturation following purification (Figure S9 A-C). As soon as 2 weeks following purification, OPTN(E50K) RGCs subjected to insulin deprivation exhibited neurite deficits, while OPTN(E50K) RGCs with insulin revealed robust neurite outgrowth comparable to wild-type RGCs with or without insulin (Figure S9 D-G), as measured by soma area, neurite length, number of primary neurites and Sholl analysis (Figure S9 H-K). Insulin deprivation also decreased the mTORC1 effector pS6Ser240/244 in OPTN(E50K) RGCs (Figure S9 L-N), as well as significantly increased the level of LC3-II (Figure S9 O-Q), suggesting an imbalance of autophagy and mTORC1 in OPTN(E50K) RGCs when deprived of insulin, resulting in RGC neurite morphological deficits. Moreover, as insulin mediates glucose metabolism [59], we next determined whether the absence of insulin in the culture media would induce metabolic stress in RGCs. Our results show that RGCs subjected to insulin deprivation exhibited reduced glucose uptake (Figure S10), suggesting increased metabolic stress induced by the absence of insulin. These results supported the idea that insulin signaling is essential to promote overall RGC neurite outgrowth, and that a lack of sufficient mTOR signaling results in neurite retraction and deficient glucose uptake.
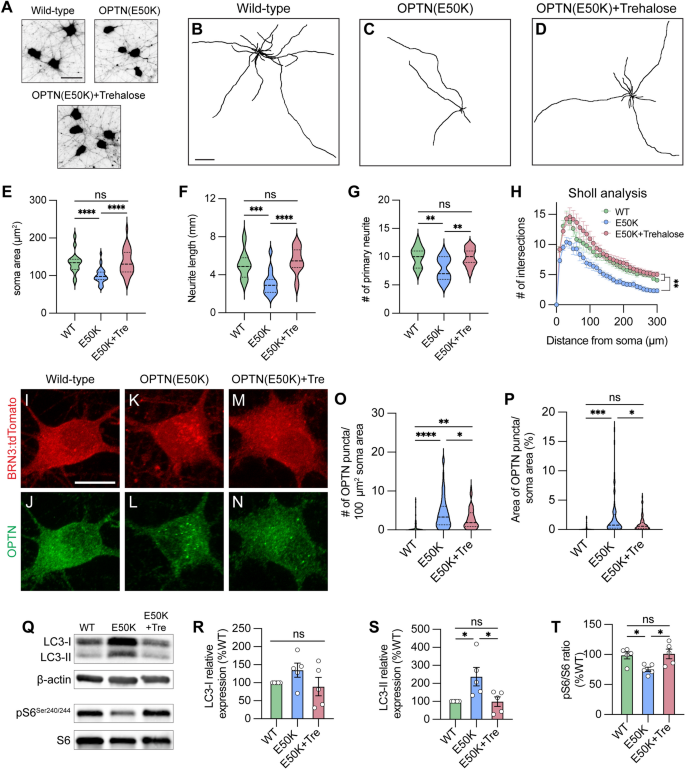
We have previously identified that autophagy deficits and the accumulation of autophagosomes can be cleared after a short term (24 h) treatment with the autophagy inducer rapamycin in OPTN(E50K) retinal organoids [12]. However, as rapamycin induces autophagy via mTOR inhibition, long term treatment with rapamycin would not represent an ideal approach due to the resulting decrease in mTOR signaling. As RGC survival relies upon the homeostatic balance between mTOR and autophagy signaling [60], we hypothesized that inducing autophagy in a manner that may also preserve mTOR signaling might rescue neurodegenerative phenotypes in OPTN(E50K) RGCs. To accomplish this, we used the compound trehalose to stimulate the autophagic-lysosomal degradation pathway in RGCs, which has been extensively characterized to function as an autophagy activator in an mTOR-independent manner [61], and has also been shown to reduce protein aggregates in a number of other neurodegenerative diseases [62,63,64,65,66]. In our experiments, OPTN(E50K) RGCs demonstrated a robust protection of neurite morphology, measured by a preservation of soma area, neurite length, number of primary neurites, and Sholl analysis following 2 weeks of trehalose treatment when compared with wild-type RGCs as well as untreated OPTN(E50K) RGCs (Fig. 7A–H). Additionally, trehalose treatment partially reduced OPTN puncta accumulation in OPTN(E50K) RGCs (Fig. 7I–P). Moreover, trehalose treatment reduced LC3-II expression as well as sustained levels of the mTORC1 effector pS6Ser240/244 (Fig. 7Q–T), collectively suggesting that treatment with trehalose can at least partially restore autophagic balance, while at the same time maintaining mTOR signaling, respectively, leading to sustained overall health of OPTN(E50K) RGCs.
mTOR-independent activator prevents neurite retraction and clears protein accumulation in OPTN(E50K) hPSC-RGCs. A Representative images of soma area in wild-type, OPTN(E50K), and trehalose-treated OPTN(E50K) hPSC-RGCs. Scale bar 25 μm. B–D Representative neurite tracing images of wild-type, OPTN(E50K), and trehalose-treated OPTN(E50K) hPSC-RGCs. Scale bar: 200 μm. E–H Quantitative analysis of neurite parameters demonstrated protection of neurites in OPTN(E50K) hPSC-RGCs after trehalose treatment for 2 weeks, as measured by the soma area (n = 3 biological replicates using WT n = 30, E50K n = 30, and E50K-trehalose n = 30 technical replicates, One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ns = not significant, p > 0.05) E, number of primary neurites (n = 3 biological replicates using WT n = 15, E50K n = 15, and E50K-trehalose n = 15 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001, ns = not significant, p > 0.05) F, total neurite length (n = 3 biological replicates using WT n = 15, E50K n = 15, and E50K-trehalose n = 15 technical replicates; One-way ANOVA, Tukey post hoc test. **p < 0.01, ns = not significant, p > 0.05) G, and Sholl analysis (n = 3 biological replicates using WT n = 15, E50K n = 15, and E50K-trehalose n = 15 technical replicates; One-way ANOVA, Tukey post hoc test. WT vs E50K: **p = 0.0095; WT vs E50K(trehalose): ns = not significant, p = 0.81; E50K vs E50K(trehalose): **p = 0.0012) H. I–N Immunostaining displayed the decrease of OPTN puncta in OPTN(E50K) hPSC-RGCs after trehalose treatment. Scale bar: 10 μm. O, P Quantification of OPTN puncta in hPSC-RGCs (n = 3 biological replicates using WT n = 51, E50K n = 60, and E50K-trehalose n = 61 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns = not significant, p > 0.05). Q–T Western blot and the relative protein expression demonstrated the recovery of changes to LC3-II and pS6Ser240/244 expression in OPTN(E50K) hPSC-RGCs after trehalose treatment. (n = 5 for each WT, E50K, and E50K with trehalose treatment; One-way ANOVA, Tukey post hoc test. LC3-I: WT vs E50K: p = 0.41, WT vs E50K(trehalose): p = 0.91, E50K vs E50K(trehalose): p = 0.23. LC3-II: WT vs E50K: p = 0.03, WT vs E50K(trehalose): p = 0.99, E50K vs E50K(trehalose): p = 0.03. pS6: WT vs E50K: p = 0.048, WT vs E50K(trehalose): p = 0.95, E50K vs E50K(trehalose): p = 0.029.). Data are all represented as mean values ± SEM
Discussion
The process of autophagy serves as a cellular protective mechanism to clear damaged proteins and organelles within the cells. Ineffective clearance of aggregated proteins in neurons contributes to neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Amyotrophic Lateral Sclerosis, and Huntington’s disease [67, 68]. Likewise, recent evidence has demonstrated that dysfunction of the autophagy degradation pathway may be involved in RGC-associated neurodegenerative diseases, including glaucoma, optic atrophy, and diabetic retinopathy [24, 60, 69]. Glaucoma is characterized by the progressive loss of RGCs, the sole type of projection neuron that connects the retina to the brain. In addition to age, elevated IOP is a prevalent risk factor for glaucoma, while a subpopulation of patients develop glaucoma due to monogenic mutations in an IOP-independent manner. Importantly, three monogenic risk genes have been identified in primary open-angle glaucoma (POAG) patients: MYOC, OPTN, and TBK1 [27]. While these genes account for approximately 5% of POAG patients [70], two of them (OPTN and TBK1) play prominent roles in autophagy. Indeed, mutations in OPTN or TBK1 can lead to glaucomatous neurodegeneration without elevated IOP, indicating that autophagy dysfunction could contribute to RGC degeneration. Here, we first examined how the OPTN(E50K) mutation contributes to autophagy deficits, resulting in RGC neurodegeneration. We used hPSC-derived RGCs as we have previously demonstrated that OPTN(E50K) hPSC-RGCs exhibited morphological and functional deficits compared to isogenic controls, supporting the idea that these cells can serve as an appropriate model to explore RGC neurodegenerative events [12]. We identified that this mutation resulted in autophagy disruption based upon increased levels of LC3-II as well as changes in autophagic flux in RGCs with the OPTN(E50K) mutation. Through the use of an LC3-RFP-GFP sensor, we noted a decreased efficiency of autophagosome and lysosome fusion, suggesting that defective autophagy led to an inability to clear protein accumulation, resulting in an elevation of metabolic stress in RGCs. Importantly, we also explored whether changes in autophagy could be observed more broadly in a magnetic microbead occlusion mouse model, thereby conferring relevance of these results more broadly across glaucoma models. Interestingly, similar to our observations in OPTN(E50K) hPSC-RGCs, we also observed increased LC3-II and changes to autophagic flux in ocular hypertensive retinas, as well as increased levels of LC3 and LAMP1 specifically within RGCs when compared to sham-operated controls. While we observed less robust autophagy disruption in a mouse model with ocular hypertension compared with human hPSC-RGCs, this could be due to differences between analyses of whole retina for the mouse model compared to purified hPSC-RGCs. While not a direct comparison, the analysis of whole retina has been performed for western blots in previous studies to examine RGCs [24, 49, 58], and the detection of significant differences despite other retinal cell types present in samples underscores the likely robustness of these phenotypes in RGCs. Collectively, our findings support the idea that autophagy dysfunction may be broadly applicable across multiple stressors associated with glaucoma, and highlight the concept that the maintenance of autophagy homeostasis is critical for RGC health by employing two species (human and mouse) as well as different risk factors (gene mutation and intraocular pressure) in glaucoma.
During the process of autophagy, OPTN interacts with LC3 to drive autophagosome maturation [29]. Multiple studies have suggested that changes to OPTN are associated with the appearance of inclusion bodies and promote neurotoxicity in various neurodegenerative diseases [28, 71,72,73,74,75,76,77]. On the contrary, the knockout of OPTN leads to deficits in ubiquitin binding or immune failure, resulting in neural damage [78,79,80]. While mutations of OPTN in the LC3-interacting region (LIR) as well as the ubiquitin binding domain (UBD) are known to alter OPTN-associated autophagy [57, 81], the E50K mutation is neither located in the LIR nor UBD. In our studies, we did not observe any differences in the expression of the ubiquitin protein between isogenic control and OPTN(E50K) RGCs, however our proteomics data did indicate misregulation of some ubiquitination-associated proteins, suggesting the possibility that altered ubiquitination may play a role in processing of mutant OPTN protein. Enhanced OPTN binding to TBK1 and increased production of insoluble OPTN has been previously observed in E50K mutant cells, suggesting that a loss of OPTN function or a gain of toxic function likely contributes to disease pathogenesis [82, 83].
In animal models, transgenic mice that express the human OPTN(E50K) transgene exhibited RGC loss and axonal degeneration [84], while another study using transgenic mice with OPTN(E50K) overexpression revealed an increased degradation of mitochondria characterized by greater induced mitophagy, resulting in RGC loss [85]. Interestingly, a more recent study demonstrated in mouse models that the OPTN(E50K) mutation decreased visual acuity, whereas visual acuity was normal in OPTN knockout mice, suggesting that the OPTN(E50K) mutation results in a toxic gain of function mechanism [86]. Correspondingly, in our studies, the robust presence of OPTN and LC3 puncta was observed in RGCs but not as much in cortical neurons, correlating with the cell type affected by this mutation in glaucoma. Secondly, clearance of OPTN puncta by treatment with trehalose rescued degenerative phenotypes in OPTN(E50K) RGCs, further supporting the idea that the OPTN(E50K) mutation results in a toxic gain of function in human RGCs as well. While OPTN(E50K) genetically modified cell lines or animals do share certain disease-responding mechanisms with human patients, the presence of the wild-type OPTN gene in the genome cannot be excluded in those models. More so, increased levels of OPTN, with or without the E50K mutation, can lead to toxic effects suggesting dose-dependent effects [87]. As a result, hPSC-RGCs generated through CRISPR/Cas9 gene editing strategies used here are likely a more suitable model to study the functional effects of the OPTN(E50K) mutation in RGCs, mimicking the cellular events that are comparable to patient RGCs that harbor the OPTN(E50K) mutation. To study whether the E50K mutation alters OPTN function, we first characterized its protein expression and localization. Intriguingly, we identified a decreased level of OPTN protein in RGCs with the E50K mutation, in contrast to other studies that overexpressed the mutant protein, suggesting that endogenous OPTN(E50K) protein may be degraded by the ubiquitin-proteosome system. This reduction of OPTN protein also seems to attenuate the recruitment of LC3 during autophagy, suggesting a loss of protein function in RGCs with the OPTN(E50K) mutation. However, whether the decreased recruitment of LC3 by OPTN directly contributes to defects in the autophagy pathway, or if this recruitment of LC3 can be compensated for by other autophagy receptors is still undetermined [88].
Functional deficits in RGCs due to the OPTN(E50K) mutation can be due to either a loss of protein function, or by the mutated protein’s gain of a toxic function through the accumulation of OPTN(E50K) protein aggregates. While we observed that the total level of OPTN protein was decreased in RGCs with the E50K mutation, an accumulation of OPTN aggregates was found in OPTN(E50K) RGCs, indicating that residual OPTN(E50K) protein can still lead to protein accumulation that may be toxic to RGCs. It is unclear if the reduction in mutant OPTN protein is sufficient to account for all observed changes, although our proteomics data (Fig. 1D) suggests that the expression of other autophagy proteins is also affected, suggesting other changes are involved. Importantly, our results comparing RGCs to cortical neurons suggests that the accumulation of OPTN(E50K) protein was unique to RGCs, which may then contribute to selective degeneration of RGCs, despite the fact that OPTN plays a role in autophagy within many cell types. Furthermore, by comparing wild-type hPSC-derived neurons, we also observed a higher level of LC3-I in RGCs than in cortical neurons, suggesting that RGCs seem to have a higher demand for autophagosome formation, and that RGCs with the OPTN(E50K) mutation cannot satisfy this requirement, leading to neurodegeneration (Fig. 5J, L). It is also important to note that the findings of the current study did not compare RGCs to other types of projection neurons, such as motor neurons. Certain missense mutations in OPTN can cause glaucoma in RGCs, whereas other mutations in OPTN, including deletions, missense, or nonsense mutations, lead to motor neuron loss in ALS [57]. The glaucoma associated mutations do not overlap with ALS associated mutations, and why these mutations selectively promote either RGC or motor neuron degeneration remains unknown. We speculate that one potential reason that the E50K mutation selectively promotes RGC loss can be linked to mitophagy. Since OPTN acts as a receptor particularly for mitophagy, a form of autophagy that selectively degrades unnecessary mitochondria, human RGCs rely heavily on mitochondria for energy supply in the optic nerve head (ONH) as well as the proximal axonal regions prior to the ONH, where RGC axons remain unmyelinated [17]. In our studies, we observed the differential expression of a number of mitochondrial-associated proteins (Figure S6), suggesting some degree of mitochondrial dysfunction due to the OPTN(E50K) mutation. This presumptive mitochondrial dysfunction could induce metabolic stress, leading to downregulation of metabolic pathways such as mTOR signaling in OPTN(E50K) RGCs, providing numerous opportunities for future investigations.
Our data show that autophagy-induced stress results in changes to the upstream regulator of autophagy, mTORC1. mTORC1 serves as a central nutrient sensor that controls protein synthesis as well as organelle degradation through autophagy during development as well as aging [89]. mTOR inhibition has been shown to only minimally activate autophagy in primary hippocampal neurons, and tau phosphorylation and Aβ metabolism likely hyperactivate mTOR in Alzheimer’s disease and Down syndrome, indicating that disruption of autophagy in Alzheimer’s disease-related neurodegenerations is mTOR-independent [90,91,92]. On the contrary, in RGCs, we and others have demonstrated that mTOR inactivation not only regulated autophagy, but also induced neurite shortening during RGC injury, while additional mTOR stimulation can trigger RGC dendrite and axon regeneration [49, 50, 58, 60, 93, 94], suggesting that mTOR activation is essential for RGC survival. Our previous findings demonstrated neurite retraction in OPTN(E50K) hPSC-RGCs, an indication of protein synthesis attenuation by reduced mTOR signaling, as well as downregulation of mTOR associated pathways from our prior RNA-seq data [12], suggesting that the OPTN(E50K) mutation altered mTOR activity in RGCs. To further confirm this finding, we assessed mTOR effectors and found that OPTN(E50K) hPSC-RGCs selectively downregulated mTORC1 and upregulated AMPK, another nutrient sensor that is activated by energy stress and serves as a negative regulator of mTORC1. However, whether AMPK regulates autophagy directly or indirectly through suppression of mTORC1 still needs to be determined [95, 96]. Interestingly, the hypoactivation of mTORC1 and upregulation of AMPK was also identified in the magnetic microbead occlusion mouse glaucoma model [58], further supporting the idea that RGC viability is mTOR-dependent.
Rapamycin and other mTOR antagonists are well-known autophagy inducers that promote clearance of protein aggregates through the inhibition of the mTOR signaling pathway, thereby removing the inhibition upon autophagy [97]. We and others have demonstrated that short term rapamycin treatment is able to clear accumulated autophagosomes and prevent cells from degenerating [12, 98]. Nevertheless, since mTOR plays a pivotal role in RGC function as discussed above, long-term exposure to rapamycin may induce adverse effects such as axonal and dendritic degeneration. In addition, it was critical to identify other means to induce autophagy to degrade OPTN(E50K) protein accumulation that did not adversely affect mTOR signaling. To this end, we choose trehalose as a means to modulate autophagy through mTOR-independent means, as trehalose is thought to induce Transcription Factor EB (TFEB) nuclear translocation and activation of autophagy-associated proteins independent to mTOR [99, 100]. We observed that trehalose improved autophagy deficits and also rescued neurite retraction in OPTN(E50K) hPSC-RGCs, supporting the idea that mTOR-independent autophagy induction could be a therapeutic target for RGC neurodegeneration by inducing autophagy while maintaining mTOR signaling to promote RGC survival. Taken together, the results of our studies demonstrate a strong connection between autophagy disruption and mTORC1 inactivation, which contributed to neurodegeneration in glaucoma.
Conclusions
The results of this study identify the OPTN(E50K) mutation as a potent inducer of protein accumulation and autophagic deficits leading to selective neurite shortening by downregulation of mTORC1 signaling in hPSC-RGCs. The dysregulation of autophagy and mTORC1 was also a consequence of RGC neurodegeneration in the microbead occlusion model, suggesting that the homeostasis of RGCs depends on this signaling pathway. While autophagy activation in RGCs is mTOR-dependent, the manipulation of autophagy through mTOR-independent strategies can rescue neurodegenerative phenotypes, providing a potential therapeutic target to prevent RGC neurodegeneration associated with glaucoma.
Availability of data and materials
Data pertaining to this study that is not directly available within the article will be made available. from the corresponding author upon reasonable request. For proteomics analysis of stem cell-derived RGCs, all processed and raw data are uploaded to ProteomeXchange Accession PXD033173.
References
Erskine L, Herrera E (2014) Connecting the retina to the brain. ASN Neuro 6(6):1759091414562107
Murcia-Belmonte V, Erskine L (2019) Wiring the binocular visual pathways. Int J Mol Sci 20(13):3282
Liton PB et al (2023) Autophagy in the eye: from physiology to pathophysology. Autophagy Rep 2(1):2178996
Nettesheim A et al (2020) Autophagy in the aging and experimental ocular hypertensive mouse model. Invest Ophthalmol Vis Sci 61(10):31
Zhang S et al (2021) The E50K optineurin mutation impacts autophagy-mediated degradation of TDP-43 and leads to RGC apoptosis in vivo and in vitro. Cell Death Discov 7(1):49
Bringmann A et al (2018) The primate fovea: structure, function and development. Prog Retin Eye Res 66:49–84
Masri RA et al (2019) Survey of retinal ganglion cell morphology in marmoset. J Comp Neurol 527(1):236–258
Peng YR et al (2019) Molecular classification and comparative taxonomics of foveal and peripheral cells in primate retina. Cell 176(5):1222-1237.e22
Huang KC, Gomes C, and. Meyer J.S, (2023) Retinal ganglion cells in a dish: current strategies and recommended best practices for effective in vitro modeling of development and disease. Handb Exp Pharmacol
Gomes C et al (2022) Astrocytes modulate neurodegenerative phenotypes associated with glaucoma in OPTN(E50K) human stem cell-derived retinal ganglion cells. Stem Cell Reports 17(7):1636–1649
Ohlemacher SK et al (2016) Stepwise differentiation of retinal ganglion cells from human pluripotent stem cells enables analysis of glaucomatous neurodegeneration. Stem Cells 34(6):1553–1562
VanderWall KB et al (2020) Retinal ganglion cells with a glaucoma OPTN(E50K) mutation exhibit neurodegenerative phenotypes when derived from three-dimensional retinal organoids. Stem Cell Reports 15(1):52–66
Teotia P et al (2017) Modeling glaucoma: retinal ganglion cells generated from induced pluripotent stem cells of patients with SIX6 risk allele show developmental abnormalities. Stem Cells 35(11):2239–2252
Sluch VM et al (2017) Enhanced stem cell differentiation and immunopurification of genome engineered human retinal ganglion cells. Stem Cells Transl Med 6(11):1972–1986
Faravelli I et al (2020) Back to the origins: human brain organoids to investigate neurodegeneration. Brain Res 1727:146561
Bowles K R, et al. (2021), ELAVL4, splicing, and glutamatergic dysfunction precede neuron loss in MAPT mutation cerebral organoids. Cell 184(17): 4547–4563 e17
Wareham LK et al (2022) Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener 17(1):23
Yu WH et al (2005) Macroautophagy–a novel beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol 171(1):87–98
Cuervo AM et al (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305(5688):1292–1295
Barmada SJ et al (2014) Autophagy induction enhances TDP43 turnover and survival in neuronal ALS models. Nat Chem Biol 10(8):677–685
Wong YC, Holzbaur EL (2015) Autophagosome dynamics in neurodegeneration at a glance. J Cell Sci 128(7):1259–1267
Porter K et al (2015) Autophagic dysregulation in glaucomatous trabecular meshwork cells. Biochim Biophys Acta 1852(3):379–385
Porter KM, Jeyabalan N, Liton PB (2014) MTOR-independent induction of autophagy in trabecular meshwork cells subjected to biaxial stretch. Biochim Biophys Acta 1843(6):1054–1062
Hirt J et al (2018) Contribution of autophagy to ocular hypertension and neurodegeneration in the DBA/2J spontaneous glaucoma mouse model. Cell Death Discov 4:14
Rezaie T et al (2002) Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 295(5557):1077–1079
Aung T et al (2005) Clinical features and course of patients with glaucoma with the E50K mutation in the optineurin gene. Invest Ophthalmol Vis Sci 46(8):2816–2822
Ritch R et al (2014) TBK1 gene duplication and normal-tension glaucoma. JAMA Ophthalmol 132(5):544–548
Osawa T et al (2011) Optineurin in neurodegenerative diseases. Neuropathology 31(6):569–574
Wong YC, Holzbaur EL (2014) Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci USA 111(42):E4439–E4448
Ying H, Yue BY (2012) Cellular and molecular biology of optineurin. Int Rev Cell Mol Biol 294:223–258
Fligor CM et al (2020) Differentiation of retinal organoids from human pluripotent stem cells. Methods Cell Biol 159:279–302
Meyer JS et al (2011) Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 29(8):1206–1218
Ohlemacher, S.K., et al. (2015) Generation of highly enriched populations of optic vesicle-like retinal cells from human pluripotent stem cells. Curr Protoc Stem Cell Biol 32: 1H 8 1–1H 8 20
Harkin J et al (2024) A highly reproducible and efficient method for retinal organoid differentiation from human pluripotent stem cells. Proc Natl Acad Sci U S A 121(25):e2317285121
Lavekar SS et al (2023) Development of a three-dimensional organoid model to explore early retinal phenotypes associated with Alzheimer’s disease. Sci Rep 13(1):13827
Gomes C et al (2024) Induction of astrocyte reactivity promotes neurodegeneration in human pluripotent stem cell models. Stem Cell Reports 19(8):1122–1136
Ito YA et al (2016) A magnetic microbead occlusion model to induce ocular hypertension-dependent glaucoma in mice. J Vis Exp 109:e53731
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta delta C(T)) Method. Methods 25(4):402–408
Pernet V, Bourgeois P, Di Polo A (2007) A role for polyamines in retinal ganglion cell excitotoxic death. J Neurochem 103(4):1481–1490
Liu X et al (2021) Proteomic analysis of aged and OPTN E50K retina in the development of normal tension glaucoma. Hum Mol Genet 30(11):1030–1044
Ying H et al (2010) Posttranslational modifications, localization, and protein interactions of optineurin, the product of a glaucoma gene. PLoS ONE 5(2):e9168
Klionsky DJ et al (2021) Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy 17(1):1–382
Evans CS, Holzbaur EL (2020) Degradation of engulfed mitochondria is rate-limiting in Optineurin-mediated mitophagy in neurons. Elife 9:e50260
Kulkarni A et al (2020) Differential regulation of autophagy during metabolic stress in astrocytes and neurons. Autophagy 16(9):1651–1667
Nixon RA, Yang DS (2012) Autophagy and neuronal cell death in neurological disorders. Cold Spring Harb Perspect Biol 4(10):a008839
Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat Med 19(8):983–997
Menzies FM, Fleming A, Rubinsztein DC (2015) Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 16(6):345–357
Kaizuka T et al (2016) An autophagic flux probe that releases an internal control. Mol Cell 64(4):835–849
Agostinone J et al (2018) Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 141(7):1963–1980
Duan X et al (2015) Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron 85(6):1244–1256
Hosokawa N et al (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20(7):1981–1991
Ganley IG et al (2009) ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem 284(18):12297–305
Proietti Onori M et al (2021) RHEB/mTOR hyperactivity causes cortical malformations and epileptic seizures through increased axonal connectivity. PLoS Biol 19(5):e3001279
Jung S et al (2019) Buffering of cytosolic calcium plays a neuroprotective role by preserving the autophagy-lysosome pathway during MPP(+)-induced neuronal death. Cell Death Discov 5:130
Yang Y et al (2020) Lysosomal dysfunction and autophagy blockade contribute to autophagy-related cancer suppressing peptide-induced cytotoxic death of cervical cancer cells through the AMPK/mTOR pathway. J Exp Clin Cancer Res 39(1):197
Jefferies HB et al (1997) Rapamycin suppresses 5’TOP mRNA translation through inhibition of p70s6k. EMBO J 16(12):3693–3704
Swarup G, Sayyad Z (2018) Altered functions and interactions of glaucoma-associated mutants of optineurin. Front Immunol 9:1287
Belforte N et al (2021) AMPK hyperactivation promotes dendrite retraction, synaptic loss, and neuronal dysfunction in glaucoma. Mol Neurodegener 16(1):43
Chang L, Chiang SH, Saltiel AR (2004) Insulin signaling and the regulation of glucose transport. Mol Med 10(7–12):65–71
Madrakhimov SB et al (2021) mTOR-dependent dysregulation of autophagy contributes to the retinal ganglion cell loss in streptozotocin-induced diabetic retinopathy. Cell Commun Signal 19(1):29
Sarkar S et al (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282(8):5641–5652
Castillo K et al (2013) Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy 9(9):1308–1320
Du J et al (2013) Trehalose rescues Alzheimer’s disease phenotypes in APP/PS1 transgenic mice. J Pharm Pharmacol 65(12):1753–1756
Li Y et al (2015) Trehalose decreases mutant SOD1 expression and alleviates motor deficiency in early but not end-stage amyotrophic lateral sclerosis in a SOD1-G93A mouse model. Neuroscience 298:12–25
Tanaka M et al (2004) Trehalose alleviates polyglutamine-mediated pathology in a mouse model of huntington disease. Nat Med 10(2):148–154
Zhang X et al (2014) MTOR-independent, autophagic enhancer trehalose prolongs motor neuron survival and ameliorates the autophagic flux defect in a mouse model of amyotrophic lateral sclerosis. Autophagy 10(4):588–602
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42
Singh K et al (2012) Autophagy-dependent senescence in response to DNA damage and chronic apoptotic stress. Autophagy 8(2):236–251
White KE et al (2009) OPA1 deficiency associated with increased autophagy in retinal ganglion cells in a murine model of dominant optic atrophy. Invest Ophthalmol Vis Sci 50(6):2567–2571
Qassim A, Siggs OM (2020) Predicting the genetic risk of glaucoma. Biochemist 42(5):26–30
Maruyama H et al (2010) Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465(7295):223–226
Schwab C et al (2012) Optineurin in Huntington’s disease intranuclear inclusions. Neurosci Lett 506(1):149–154
Mori F et al (2012) Optineurin immunoreactivity in neuronal nuclear inclusions of polyglutamine diseases (Huntington’s, DRPLA, SCA2, SCA3) and intranuclear inclusion body disease. Acta Neuropathol 123(5):747–749
Shen WC et al (2015) Mutations in the ubiquitin-binding domain of OPTN/optineurin interfere with autophagy-mediated degradation of misfolded proteins by a dominant-negative mechanism. Autophagy 11(4):685–700
Sirohi K, Swarup G (2016) Defects in autophagy caused by glaucoma-associated mutations in optineurin. Exp Eye Res 144:54–63
Anborgh PH et al (2005) Inhibition of metabotropic glutamate receptor signaling by the huntingtin-binding protein optineurin. J Biol Chem 280(41):34840–34848
De Marco N et al (2006) Optineurin increases cell survival and translocates to the nucleus in a Rab8-dependent manner upon an apoptotic stimulus. J Biol Chem 281(23):16147–16156
Munitic I et al (2013) Optineurin insufficiency impairs IRF3 but not NF-kappaB activation in immune cells. J Immunol 191(12):6231–6240
Slowicka K et al (2016) Optineurin deficiency in mice is associated with increased sensitivity to Salmonella but does not affect proinflammatory NF-kappaB signaling. Eur J Immunol 46(4):971–980
Markovinovic A et al (2018) Optineurin Insufficiency Disbalances Proinflammatory and Anti-inflammatory Factors by Reducing Microglial IFN-beta Responses. Neuroscience 388:139–151
Qiu Y et al (2021) Emerging views of OPTN (optineurin) function in the autophagic process associated with disease. Autophagy 18:1–13
Li F et al (2016) Structural insights into the interaction and disease mechanism of neurodegenerative disease-associated optineurin and TBK1 proteins. Nat Commun 7:12708
Minegishi Y et al (2013) Enhanced optineurin E50K-TBK1 interaction evokes protein insolubility and initiates familial primary open-angle glaucoma. Hum Mol Genet 22(17):3559–3567
Tseng HC et al (2015) Visual impairment in an optineurin mouse model of primary open-angle glaucoma. Neurobiol Aging 36(6):2201–2212
Shim MS et al (2016) Mitochondrial pathogenic mechanism and degradation in optineurin E50K mutation-mediated retinal ganglion cell degeneration. Sci Rep 6:33830
Su CC et al (2024) Age-related effects of optineurin deficiency in the mouse eye. Vision Res 224:108463
Park BC et al (2006) Studies of optineurin, a glaucoma gene: Golgi fragmentation and cell death from overexpression of wild-type and mutant optineurin in two ocular cell types. Am J Pathol 169(6):1976–1989
Shoemaker CJ et al (2019) CRISPR screening using an expanded toolkit of autophagy reporters identifies TMEM41B as a novel autophagy factor. PLoS Biol 17(4):e2007044
Kim DH et al (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110(2):163–175
Maday S, Holzbaur EL (2016) Compartment-specific regulation of autophagy in primary neurons. J Neurosci 36(22):5933–5945
Querfurth H, Lee HK (2021) Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol Neurodegener 16(1):44
Linda K et al (2022) Imbalanced autophagy causes synaptic deficits in a human model for neurodevelopmental disorders. Autophagy 18(2):423–442
Park KK et al (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322(5903):963–966
Teotia P et al (2019) Human retinal ganglion cell axon regeneration by recapitulating developmental mechanisms: effects of recruitment of the mTOR pathway. Development 146(13):dev178012
Inoki K, Kim J, Guan KL (2012) AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol 52:381–400
Ji MM et al (2015) Induction of autophagy by valproic acid enhanced lymphoma cell chemosensitivity through HDAC-independent and IP3-mediated PRKAA activation. Autophagy 11(12):2160–2171
Guertin DA, Sabatini DM (2009) The pharmacology of mTOR inhibition. Sci Signal 2(67):pe24
Chalasani ML et al (2014) E50K-OPTN-induced retinal cell death involves the Rab GTPase-activating protein, TBC1D17 mediated block in autophagy. PLoS ONE 9(4):e95758
Rusmini P et al (2019) Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 15(4):631–651
Palmieri M et al (2017) mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat Commun 8:14338
Acknowledgements
We thank Peter Bor-Chian Lin and Adrian Oblak for sharing reagents, as well as Mallika Valapala for helping discussions regarding trehalose experiments. We also thank Amelia Linneman for helpful discussions about relevant autophagy assays, Amber Mosley and Emma Doud at the IU School of Medicine Center for Proteome Analysis for assistance with proteomics experiments, and the IU School of Medicine Flow Cytometry Core Facility for assistance with cell sorting.
Funding
Grant support was provided by the National Eye Institute (R01EY033022 and U24EY033269 to JSM), the BrightFocus Foundation (G2020369 to JSM and G2022003F to CG), the Gilbert Family Foundation (923016 to JSM), the Glaucoma Research Foundation (to JSM), and the Indiana Department of Health Spinal Cord and Brain Injury Research Fund (26343 to JSM). Support for this project was also provided by the Sarah Roush Memorial Fellowship from the Indiana Alzheimer’s Disease Center (CG). This publication was also made possible with partial support from the Stark Neurosciences Research Institute/Eli Lilly and Co. Neurodegeneration fellowship (KCH), from a pre-doctoral fellowship award (KBV) from the Indiana CTSI and a core pilot award for use of the IUSM Proteomics Core (both funded by the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (UL1TR002529, A. Shekhar, PI)), and from Sigma Xi Grants in Aid of Research (G03152021117541788 to KCH). This work was also partially funded by a grant to ADP from the National Institutes of Health (NIH, 1R01EY030838-01). YS was supported by postdoctoral fellowships from the Fonds de recherche Santé – Québec (FRQS, 276181) and the Canadian Institiutes of Health Research (CIHR, 458569).
Author information
Authors and Affiliations
Contributions
KCH and JSM conceived the project and designed the experiments. KCH, CG, YS, NB, KBV, SSL, CMF, JH collected samples. KCH, CG, YS, SMH, SVP performed experiments and/or analyzed results. KCH, CG, YS, ADP, and JSM wrote and edited the manuscript. KCH, CG, YS, KBV, ADP, JSM secured funding associated with this project. ADP, JSM supervised these studies. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments outlined within this manuscript were performed according to either IBC protocols.approved by Indiana University or animal use (IACUC) protocols approved by the University of Montreal.
Consent for publication
Not applicable.
Competing interests
JSM holds a patent related to methods for the retinal differentiation of human pluripotent stem cells used in this study, and ADP holds a Canada Research Chair (Tier 1).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, KC., Gomes, C., Shiga, Y. et al. Acquisition of neurodegenerative features in isogenic OPTN(E50K) human stem cell-derived retinal ganglion cells associated with autophagy disruption and mTORC1 signaling reduction. acta neuropathol commun 12, 164 (2024). https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s40478-024-01872-2
Received:
Accepted:
Published:
DOI: https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s40478-024-01872-2